Projects in the lab are always changing, please contact the PI for the most up to date opportunities. To give a favor of the types of projects, a few examples are listed here:
Identifying novel glial therapeutic targets for Parkinson’s disease
The genetic tools available in Drosophila make it possible to knockdown a large number of genes in glia in order to identify those that can rescue neuronal alpha-synuclein toxicity. We have screened hundreds of genes to date and have many promising targets for follow up. We are currently doing a screen of the “druggable” proteome, a list of ~600 genes that code for proteins that are targets of FDA-approved drugs.

Unpacking gene-environment interactions in Parkinson’s disease
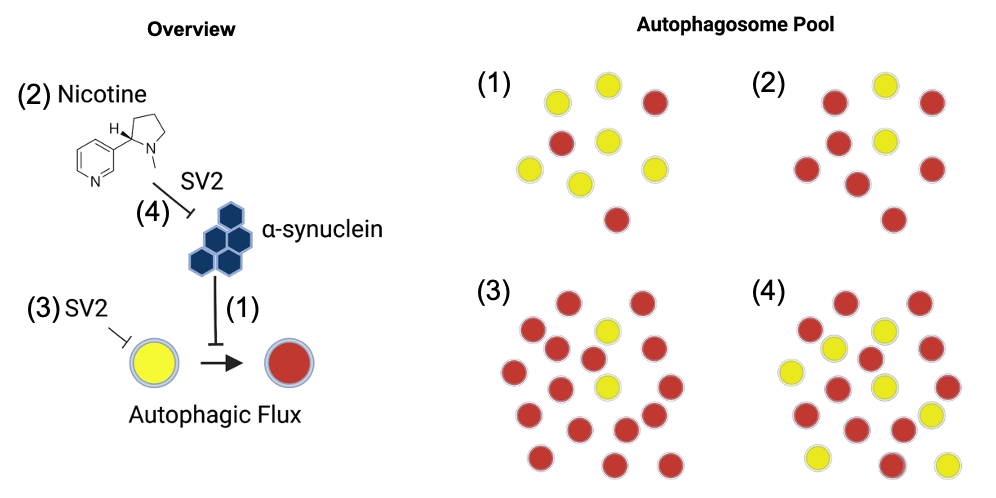
Genetics explain less than ~30% of the risk of Parkinson’s disease, meaning that environmental factors play a large role in risk. Factors such as pesticide use, head trauma, smoking, and caffeine intake all influence the risk of development of Parkinson’s, and they likely interact with genetic risk in complex ways. We are interested in modeling these gene-environment interactions in animals and have started with nicotine, identifying a gene-environment interaction between nicotine and SV2C and a novel role for this interaction in autophagy. We are now seeking to understand the mechanism of this in more detail.
Developing animal models for non-motor systems of Parkinson’s disease
Parkinson’s disease is defined by its motor symptoms (bradykinesia, tremor, and rigidity), but it is actually non-motor symptoms that cause the greatest impairment in quality of life for patients. It may not sound very glamorous, but constipation is a big problem for patients! We have developed a new Drosophila model for studying the neuroanatomy of constipation. By understanding which cell types and pathways are involved, we hope to eventually develop new therapies for this intractable problem. We are taking a similar approach to understand other non-motor symptoms, such as olfactory loss, sleep disturbances, and cognitive impairment. These symptoms are also particularly relevant to other diseases in the Parkinson’s disease family, such as multiple system atrophy and dementia with Lewy bodies.


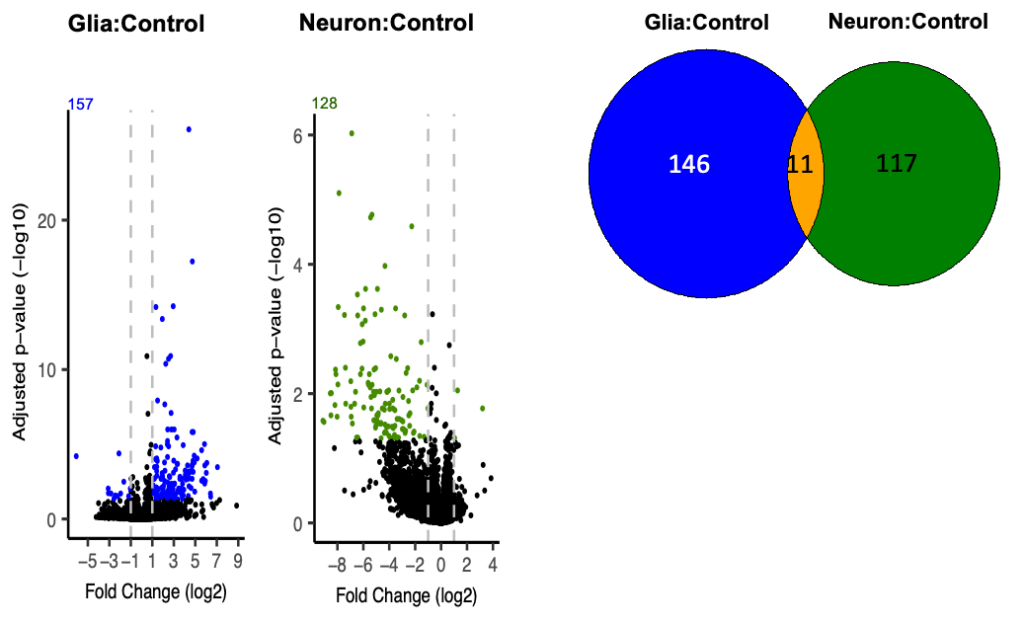
Understanding alpha-synuclein induced gene transcription
Alpha-synuclein inclusions are found not only in neurons but also in glial cells, particularly in multiple system atrophy (MSA), in which the defining pathologic feature is the glial cytoplasmic inclusion. The effects of alpha-synuclein in glia are poorly understood. We expressed alpha-synuclein in glia or neurons in Drosophila, finding that it has very different effects on gene transcription in the whole brain depending on the cell type in which it is expressed. We are now seeking to understand the mechanism underlying these differences.